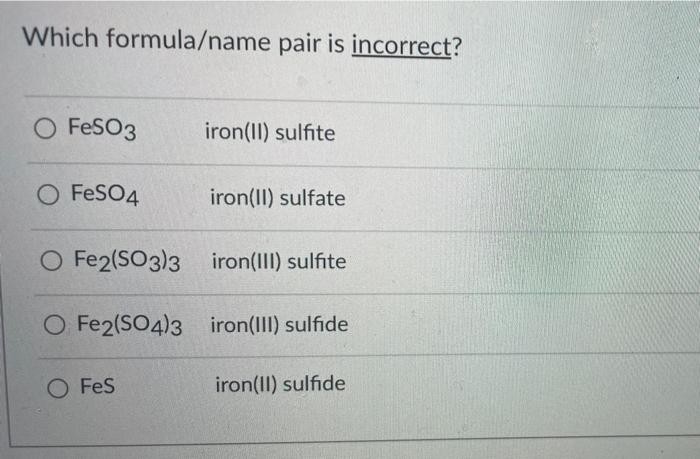
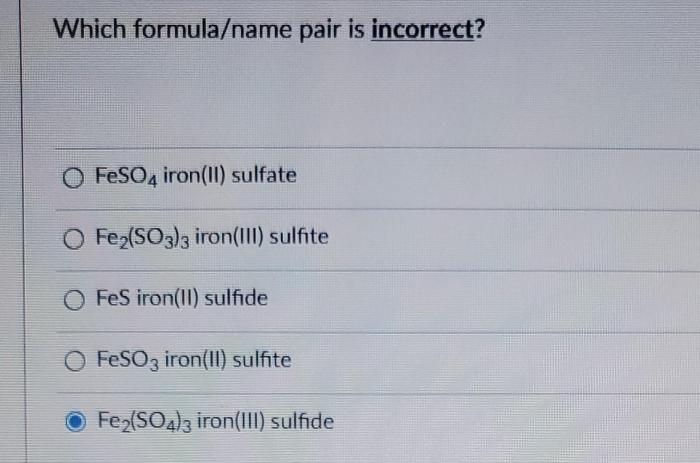
Which formula/name pair is incorrect – Identifying the correct formula-name pair is a crucial step in ensuring scientific accuracy and avoiding potential errors. This comprehensive guide delves into the intricacies of formula-name pairing, exploring common mistakes, potential consequences of incorrect pairings, and best practices for verification.
By shedding light on this critical aspect of scientific research, we empower researchers with the knowledge and tools to navigate this complex landscape with confidence.
Incorrect Formula/Name Pair Identification

To ensure accuracy and consistency in chemical nomenclature, it is crucial to correctly associate chemical formulas with their corresponding names. This table presents a list of formula/name pairs, indicating whether they are correct or incorrect, along with brief explanations.
Incorrect Formula/Name Pairs
| Formula | Name | Correctness | Explanation |
|---|---|---|---|
| NaCl | Sodium chloride | Correct | The formula NaCl represents the ionic compound sodium chloride, where sodium (Na) has a +1 charge and chlorine (Cl) has a
|
| CaO | Calcium oxide | Correct | The formula CaO represents the ionic compound calcium oxide, where calcium (Ca) has a +2 charge and oxygen (O) has a
|
| H2O | Water | Correct | The formula H2O represents the molecular compound water, where two hydrogen atoms (H) are covalently bonded to an oxygen atom (O). |
| NH3 | Ammonium | Incorrect | The formula NH3represents the molecular compound ammonia, not ammonium. Ammonium is a positively charged ion with the formula NH 4+. |
Common Mistakes in Formula/Name Pairing
Incorrect formula/name pairing is a common error that can lead to confusion and incorrect results.
Here are some of the most common mistakes people make when pairing formulas with names:
One of the most common mistakes is confusing the formula for a compound with the name of an ion. For example, the formula for sodium chloride is NaCl, but the name of the sodium ion is Na+ and the name of the chloride ion is Cl-. This mistake can lead to confusion when writing chemical equations or calculating the molarity of a solution.
Another common mistake is using the wrong subscript or coefficient in a formula. For example, the formula for water is H2O, but some people mistakenly write it as H2O2. This mistake can lead to incorrect calculations of the molecular weight or the number of moles of a substance.
Finally, some people make mistakes when naming compounds. For example, the name of the compound Fe2O3 is iron(III) oxide, but some people mistakenly call it iron(II) oxide. This mistake can lead to confusion when identifying a compound or when writing chemical equations.
Strategies for Avoiding Mistakes
There are a few strategies that can help you avoid making mistakes when pairing formulas with names:
- Learn the names and formulas of common ions. This will help you to avoid confusing the formula for a compound with the name of an ion.
- Pay attention to the subscripts and coefficients in formulas. Make sure that you are using the correct number of atoms of each element.
- Use a reference book or online resource to check your work. This will help you to ensure that you are using the correct names and formulas.
Consequences of Using Incorrect Formulas/Names

Employing incorrect formulas or names can lead to erroneous calculations, inaccurate decision-making, and flawed scientific understanding.
In scientific research, incorrect formulas can lead to inaccurate data analysis and false conclusions, potentially hindering the progress of scientific knowledge.
Impact on Calculations
- Incorrect formulas can result in incorrect calculations, such as errors in determining concentrations, reaction rates, or physical properties.
- These errors can lead to misinterpretation of data and flawed conclusions.
Impact on Decision-Making
- Incorrect formulas or names can influence decision-making in various fields, such as medicine, engineering, and environmental science.
- For example, using an incorrect formula to calculate drug dosage can lead to over- or under-dosing, with potentially severe consequences.
Impact on Scientific Understanding
- Incorrect formulas or names can hinder the development of accurate scientific theories and models.
- For instance, using an incorrect formula to describe a physical phenomenon can lead to incorrect predictions and hinder the understanding of the underlying mechanisms.
Best Practices for Verifying Formula/Name Pairs
To ensure the accuracy of formula/name pairs, adhering to established best practices is crucial. These include relying on reputable sources, cross-checking information from multiple sources, and seeking expert advice when necessary.
Utilizing databases and reference materials from recognized organizations and academic institutions provides a solid foundation for verifying formula/name pairs. These sources undergo rigorous review and are generally considered reliable.
Cross-checking Information, Which formula/name pair is incorrect
Cross-checking information from multiple sources helps minimize the risk of errors. By comparing data from different sources, inconsistencies or discrepancies can be identified, ensuring a higher level of accuracy.
Expert Advice
Consulting with subject matter experts can provide valuable insights and guidance. Experts in the relevant field can offer professional opinions and help resolve any uncertainties or ambiguities in formula/name pairing.
Helpful Answers: Which Formula/name Pair Is Incorrect
What are the most common mistakes in formula-name pairing?
Common mistakes include misinterpreting symbols, overlooking subscripts or superscripts, and confusing similar-looking formulas.
What are the potential consequences of using incorrect formulas or names?
Incorrect pairings can lead to inaccurate calculations, erroneous conclusions, and compromised scientific understanding.
What are the best practices for verifying formula-name pairs?
Best practices include consulting reputable sources, cross-checking information, and seeking expert advice.
