Construct the expression for ka for the weak acid ch3cooh – Constructing the expression for Ka for the weak acid CH3COOH is a fundamental concept in understanding the behavior of weak acids in aqueous solutions. Ka, the acid dissociation constant, plays a crucial role in predicting the extent of dissociation, pH, and other important properties of weak acid solutions.
This detailed guide will provide a step-by-step approach to constructing the expression for Ka, exploring the relationship between Ka, [H+], and [CH3COOH], and discussing the significance of Ka in various chemical and biological contexts.
Weak Acid Dissociation Constant (Ka): Construct The Expression For Ka For The Weak Acid Ch3cooh
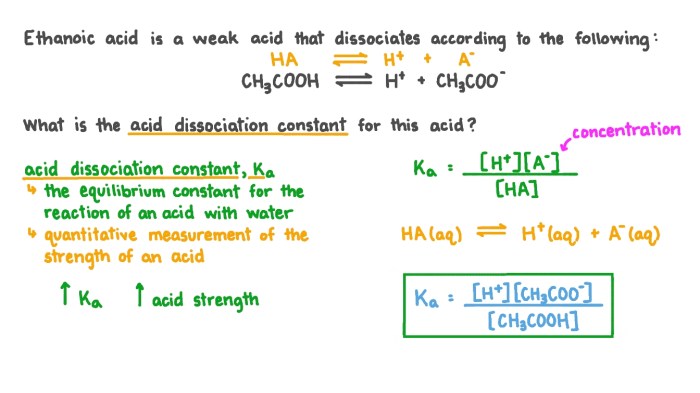
The weak acid dissociation constant (Ka) is a quantitative measure of the strength of a weak acid in water. It represents the equilibrium constant for the dissociation reaction of the acid, and it provides valuable insights into the behavior of weak acids in solution.
Equilibrium and Ka, Construct the expression for ka for the weak acid ch3cooh
Weak acids undergo a reversible dissociation reaction in water, where a proton (H+) is transferred from the acid to water molecules. This reaction establishes an equilibrium between the undissociated acid (HA) and its conjugate base (A-) and H+ ions:
HA(aq) + H2O(l) ⇌ H3O+(aq) + A-(aq)
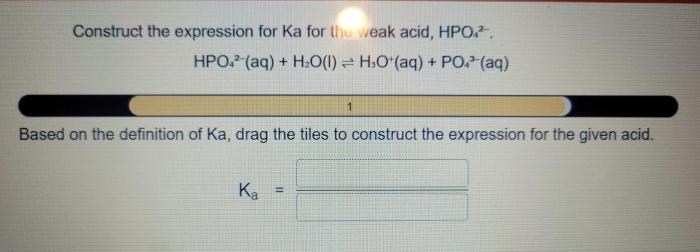
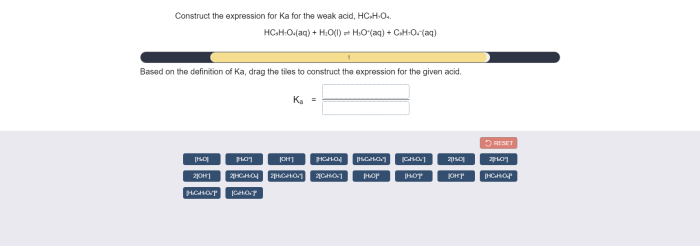
The equilibrium constant, Ka, for this reaction is defined as the ratio of the concentrations of the products to the concentration of the reactants at equilibrium:
Ka = [H3O+][A-] / [HA]
Dissociation of CH3COOH
Acetic acid (CH3COOH) is a weak acid that dissociates in water to form its conjugate base, acetate ions (CH3COO-), and H+ ions:
CH3COOH(aq) + H2O(l) ⇌ H3O+(aq) + CH3COO-(aq)
The extent of dissociation, represented by the fraction of acid molecules that dissociate, is determined by the strength of the acid and the concentration of the acid solution.
Constructing the Expression for Ka
The expression for Ka can be derived from the equilibrium constant expression for the dissociation reaction of the weak acid:
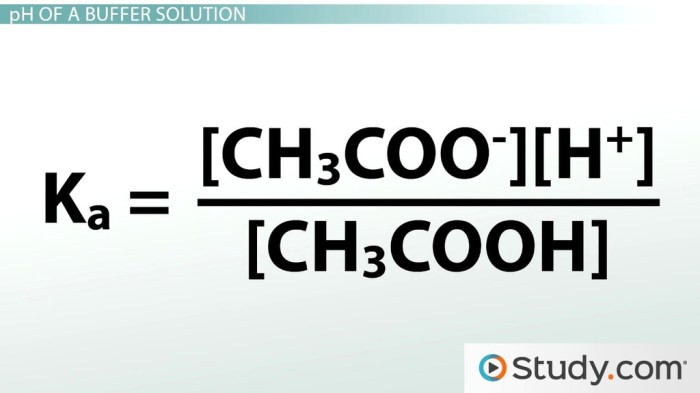
Ka = [H+][A-] / [HA]
Since the concentration of water is essentially constant, it can be included in the equilibrium constant as a new constant, Kw:
Kw = [H+][OH-] = 1.0 x 10^-14
Substituting Kw into the expression for Ka, we get:
Ka = Kw[A-] / [HA][OH-]
This equation can be rearranged to obtain the expression for Ka in terms of the concentrations of H+ and CH3COOH:
Ka = [H+][CH3COO-] / [CH3COOH]
Applications of Ka
Ka is a valuable tool in understanding the behavior of weak acids in various chemical and biological contexts. It can be used to:
- Predict the pH of weak acid solutions
- Determine the concentration of weak acids
- Investigate the influence of temperature and ionic strength on acid dissociation
- Understand the role of weak acids in biological systems, such as in maintaining pH homeostasis and enzyme catalysis
FAQ Insights
What is the significance of Ka in understanding weak acid behavior?
Ka provides a quantitative measure of the strength of a weak acid, indicating its tendency to dissociate in water. A higher Ka value corresponds to a stronger acid, which dissociates more readily.
How does the extent of dissociation relate to Ka?
The extent of dissociation is directly proportional to Ka. A higher Ka value indicates a greater extent of dissociation, resulting in a higher concentration of H+ ions and a lower concentration of undissociated acid.
What factors influence the value of Ka?
The value of Ka is influenced by the structure of the acid, solvent effects, and temperature. Structural factors such as the presence of electronegative groups or resonance can affect the stability of the conjugate base and, consequently, the Ka value.